As if climate change didn’t already come with enough adverse side effects, there’s yet another that we can add to the list: water acidification. Acid levels in oceans and freshwaters are rising, to the detriment of the sea and aquatic life. Acid levels in oceans have been increasing for several decades, and studies have shown levels of freshwater acidification has gone up as well.
So, what exactly does it mean that our waters are acidifying? Basically, as more and more pollutants are released into our atmosphere, many of those contaminants and elements end up in our water. What has scientists concerned is that higher carbon dioxide levels will hurt the balance of the aquatic food chain, among other problems.
as more and more pollutants are released into our atmosphere, many of those contaminants and elements end up in our water.
Acidifying Waters Break the Food Chain
German water researchers conducted a recent analysis of reservoir water data collected from 1981 to 2015 that revealed that aquatic CO2 levels had continuously risen in several German lakes, and found that the pH level in those water sources had increased at the rate of .01 per year on average since 1981. If Germany’s water supply sounds like a far away from that doesn’t affect the water here in the United States, welp, sorry to say this increase in acidity is far more widespread. According to a UK air quality organization, areas all around the world have been affected by a rise in acidity, including such far-flung places as Scandinavia, Central Europe, Scotland, Canada as well as the United States.
What does that mean for water bodies with lower alkaline levels? For one, it has potentially damaging implications for aquatic life, and consequently for humans. In the same German study, researchers also studied organisms called daphnia (perhaps better known by their colloquial name: water fleas) which are in abundance in freshwater bodies because they’re a significant source of food at the bottom of the food chain. The scientists found that the daphnia’s defense mechanisms for avoiding predators had weakened, and believed the increased CO2 in their test samples had interfered with the daphnia’s sense of smell, which is the defense mechanism that alerts them to predators in their midst. That’s significant because those defenses are what keeps the daphnia alive, and thus, able to perpetuate their species, and further up the food chain, other water creatures need the daphnia, which is a major food supply.
The Acid in Your Glass of Water
There are many causes of acidification of water bodies, some natural and others manmade — like changes in both water use and land use — but what they have in common is the effect of disrupting the pH balance of a water body or system. Rising carbon dioxide emissions have a huge impact on acid levels in water, as it’s not just our air that absorbs that CO2, our oceans do as well. Other culprits of acidifying waters include nitrogen fertilizers, introducing livestock into a water zone that didn’t previously have them, acid rain, and alkaline-poor soil.
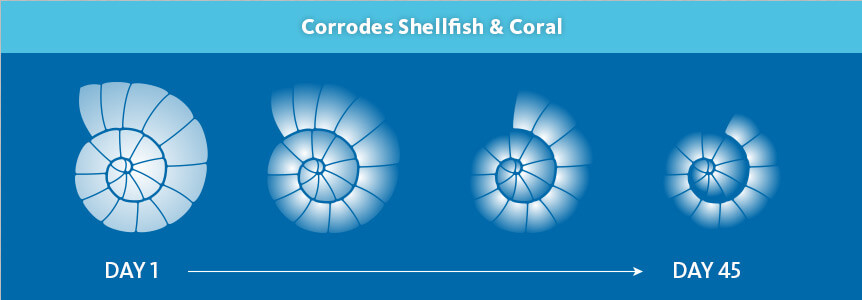
But, you may ask, how does acidification affect drinking water? Is it just turning everything into orange juice? Well, no, only oranges can do that. Acidification, on the other hand, gradually changes the aquatic life, and can upset the balance of predator/prey with the result of decreasing biodiversity. These pH changes tend to affect soft-bodied aquatic life — crayfish, snails, leeches — first. In addition, many insect species fall victim, and those healthy dinner staples, salmon and trout, are vulnerable as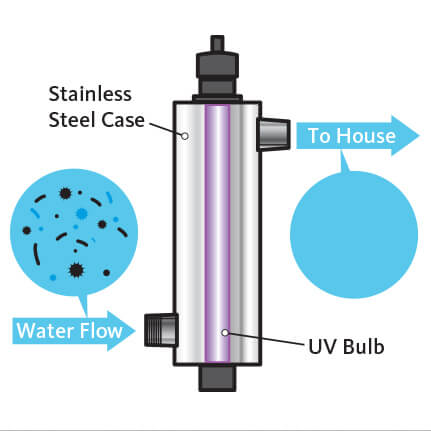 well. Increasing acid levels also interrupt the processes of calcifying sea creatures, such as coral and shellfish. So, if you love Maryland crab cakes, well, this could make that vacation a little disappointing. Meanwhile, species that are resistant to acidification grow in number, perpetuating this upset of the food chain.
well. Increasing acid levels also interrupt the processes of calcifying sea creatures, such as coral and shellfish. So, if you love Maryland crab cakes, well, this could make that vacation a little disappointing. Meanwhile, species that are resistant to acidification grow in number, perpetuating this upset of the food chain.
Of course, this begs the question: what impact does this have on humans? Well, there’s one major concern that these increasing acid levels will make fish and other dietary staples less widely available. But the impacts on good water for people are also an area of growing concern. Why? Acidifying waters release toxic metals, retain phosphorus, and (as previously mentioned) increase carbon dioxide in the water.
Re-Balancing Your Water
The most effective way to stop water acidification is to reduce carbon dioxide and sulfur emissions, and unfortunately, that’s more of a regulation and policy issue than something individuals can take direct action to change. So, call on your lawmakers to regulate carbon dioxide and sulfur emissions, as our air quality also has an effect on our water quality. According to the Food and Water Watch, another action that would help stem the tide of acidification would be addressing coastal water pollution inputs, which is another multi-pronged solution, but one way of addressing these would be demanding greater agricultural regulation. Meanwhile, if you’re concerned about your water quality and its degree of acidity, protect yourself at home with a water filtration system and a UV light. It’s one more layer of assurance and one of the few things individuals can control.
